Clinical Research
-
Facebook
-
Twitter
-
Linkedin
Explore the latest developments in cellular research, including stem cell therapy, regenerative medicine, and innovative approaches to treating diseases at the cellular level.
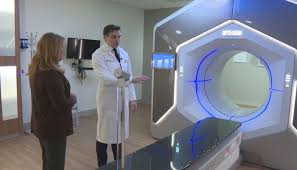
3News exclusive: Breakthrough radiation therapy at University Hospitals cuts treatment time from weeks to days
A revolutionary advancement in cancer treatment at UH Seidman Cancer Center is dramatically reducing the time patients spend receiving radiation therapy.

Breakthrough nanoparticle delivery method targets brain inflammation for multiple conditions
Oregon State University researchers have discovered a way to get anti-inflammatory medicine across the blood-brain barrier, opening the door to potential new therapies for a range of conditions, including Alzheimer’s disease, multiple sclerosis, Parkinson’s disease and cancer cachexia.

How a common diabetes drug may help the immune system fight cancer
Metformin is best known as a reliable and affordable medication for managing type 2 diabetes.

How to prime tumors to be defeated by cancer immunotherapy
Experts have thought that immune cells had to be inside of tumors for one type of immunotherapy, known as checkpoint inhibitors, to work.

What turns breast cancer cells aggressive? Chinese team may have found the key
A team of Chinese scientists has uncovered a key mechanism behind how breast cancer cells weaponise the amino acid arginine to evade the immune system and multiply.

This AI is creating cancer-killing molecules
The hunt for cancer-combatting antibodies normally relies upon human lab scientists combing through vast amounts of data. LabGenius’ automated AI robotic system has disrupted that process.

Scientists develop powerful blood test method to detect and track cancer
A new study has revealed a promising method that could one day allow doctors to detect and monitor cancer using only blood tests.
Cheap diabetes drug taken by millions of Britons could protect against deadly cancer
A diabetes drug which costs just 35p per pill could help prevent an aggressive form of blood cancer, according to University of Cambridge researchers.

Unique Stanford Medicine-designed AI predicts cancer prognoses, responses to treatment
A new artificial intelligence tool developed at Stanford Medicine combines data from medical images with text to predict cancer prognoses and treatment responses.

Reprogramming cancer cells to treat an aggressive type of leukemia
A Ludwig Cancer Research study has identified a novel strategy for treating acute myelogenous leukemia (AML), an aggressive blood cancer for which the median survival time following diagnosis remains just 8.5 months.

CARsgen’s Claudin18.2 CAR-T Therapy Satri-cel Granted Breakthrough Therapy Designation by the NMPA
SHANGHAI, March 2, 2025 /PRNewswire/ — CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) has granted Breakthrough Therapy Designation (BTD) to satricabtagene autoleucel (“satri-cel”, CT041) for the treatment of Claudin18.2-positive advanced gastric/gastroesophageal junction cancer (G/GEJ) in patients who have failed at least two prior lines of therapy.

Common antiparasitic drug shows promise in halting growth of aggressive skin cancer
A common pinworm medication may stop and reverse cancer growth in Merkel cell carcinoma, an aggressive form of skin cancer, according to research led by University of Arizona Cancer Center researchers






